Microscale 3D printing for medicine
New “jet writing” technique can make detailed 3D structures with clinically relevant materials for future implants and cancer studies.

New “jet writing” technique can make detailed 3D structures with clinically relevant materials for future implants and cancer studies.
Precision 3D structures can be built with implantable materials using a new method pioneered at the University of Michigan, and this capability could enable better cancer treatments as well as implants using stem cells to create living patches.

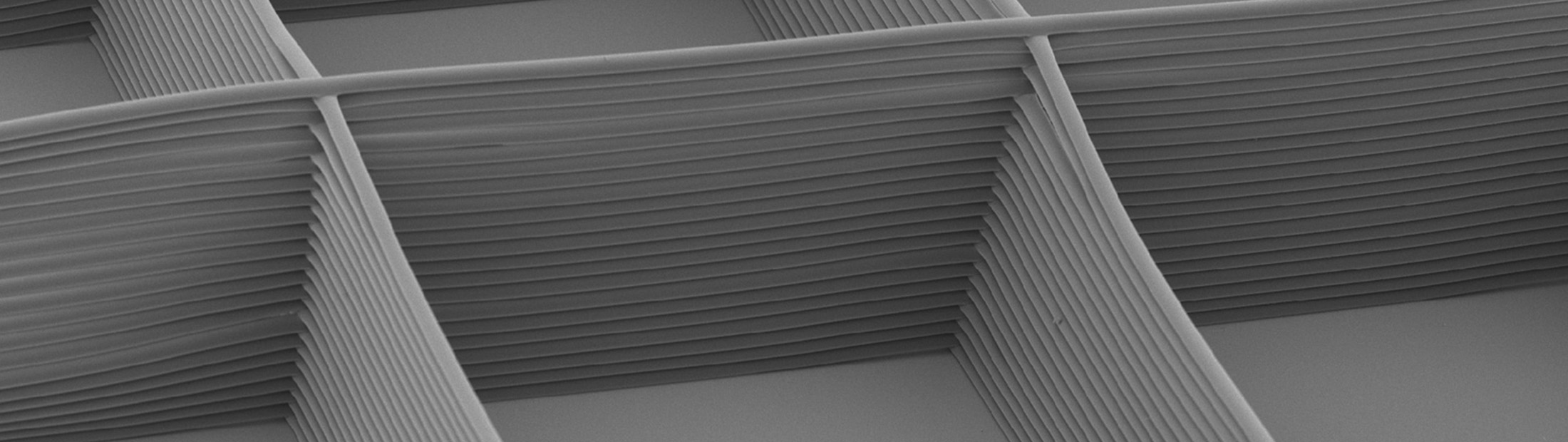
For a long time, polymers (such as plastics) have been spun into threads with diameters on the microscale by electrically charging the polymer and flowing it through a needle. This produces a thin jet that runs toward an electrical ground, but lacks control over where that thread lands. Now, by modifying the electric fields around the polymer thread, the team has achieved fine control over the jet and built the threads into a variety of lattices.
“3D jet writing enables the next step forward in tissue engineering applications including drug screening, tissue regeneration and personalized medicine,” said Joerg Lahann, professor of chemical engineering. “We can stack fibers one tenth the size of a human hair into complex 3D structures, creating housing for living cells using very little non-biological material. This is particularly important for stem cells, which turn into particular tissues based on cues in their environments.”
To prove this concept, the team created 3D-written scaffolds to patch holes in the skulls of mice. They loaded stem cells into the scaffolds and soaked them in a fluid that encouraged the cells to become bone cells. They then inserted the scaffolds into the holes in the skulls. While these holes were too big to heal on their own, they closed completely in the patched mice.
Lahann is also interested in the potential to use the scaffolds to create a tumor-like environment. Tumor cells captured from patients – either through biopsies or blood draws – could be implanted in the scaffolds. These artificial tumors could then be treated with different drug combinations, helping doctors identify the right therapy to kill off a tumor.
“3D jet writing bridges the macroscale and microscale. While the scaffold itself is large enough to handle, the features within it are on the scale of a cell. This provides an environment where cells can grow in 3D alongside other cells, creating a more accurate tissue mimic,” said Jacob Jordahl, the inventor of 3D jet writing. “Whether this tissue is anything from cancer to bone, 3D jet writing can be implemented to answer a diverse range of research questions.”
Cells can grow in 3D alongside other cells, creating a more accurate tissue mimic.
JACOB JORDAHL
PhD STUDENT in chemical engineering
Bone is a common metastatic site, so the team implanted the bone scaffolds into mice to see if the scaffolds could fool cancer cells into starting new tumors there. They then injected breast cancer cells into the mice. Indeed, the bone scaffolds soon supported colonies of breast cancer cells.
The study, titled, “3D Jet Writing: Functional Microtissues Based on Tessellated Scaffold Architectures,” is published in Advanced Materials.
This work was done in collaboration with Gary Luker, a professor of radiology and microbiology and immunology at Michigan Medicine, as well as Paul Krebsbach, dean of dentistry at the University of California Los Angeles, and was facilitated by the Biointerfaces Institute at the University of Michigan.
The study was funded by the National Institutes of Health (grant no. U01 CA210152-01A1) with additional support from the National Science Foundation and Department of Defense.
Lahann is also director of the Biointerfaces Institute as well as a professor of biomedical engineering, macromolecular science and engineering, and materials science and engineering.