
Histotripsy tumor treatment moves from trials to triumphs in 2024
U-M co-inventor of the cancer treatment has been named a National Academy of inventors fellow.

U-M co-inventor of the cancer treatment has been named a National Academy of inventors fellow.
Experts
In just over a year, histotripsy, the non-invasive ultrasound-based tumor-destroying treatment invented and developed at the University of Michigan, jumped from trial-stage hopeful to real-world therapy option that has benefited roughly 800 patients across the U.S.
And the technology is already gaining footholds in other countries.
U.S. Food and Drug Administration officials cleared the way for histotripsy treatments in humans with liver tumors in October 2023, granting approval to HistoSonics, the U-M startup founded by engineers and doctors in 2009 to commercialize the technology. That announcement catapulted it off the starting blocks and into 14 months of rapid expansion and development.
Histotripsy is now available in 18 states, the United Arab Emirates and Hong Kong. While HistoSonics takes the technology global, doctors and engineers continue advancing research on the paradigm-shifting technique and how it can benefit patients. Results from the first large clinical trial underscored histotripsy’s effectiveness for liver tumors, and new trials have been launched to explore its utility for treating additional types of cancer.
For Zhen Xu, the professor of biomedical engineering who co-invented the treatment over more than two decades of research, 2024 has been equally as eventful.
In recent weeks, she was named a fellow of the National Academy of Inventors (NAI), a fellow of the Institute of Electrical and Electronics Engineers (IEEE), and learned she would receive an endowed professorship. Next year, she will become the first Li Ka Shing Professor of Biomedical Engineering at U-M. Li, of Hong Kong,is one of the world’s richest people and a famed philanthropist. His foundation funded the endowed professorship to “support discoveries in biomedical engineering that can be translated to the benefit of humanity into the future.”

“Professor Xu’s ingenuity has led to a remarkable technology that is transforming cancer treatment for patients across the country,” said Karen Thole, the Robert J. Vlasic dean of engineering at U-M. “I’m incredibly proud of the impacts histotripsy is having and the robust University of Michigan ecosystem that enabled it. This is just the start of potential applications that will save lives.”
Histotripsy is helping more people each day and the professional recognition that has come as a result is gratifying, Xu says. Yet she feels a sense of frustration.
“I hate that I’m not moving fast enough,” she said. “It’s all about helping as many people as we can as soon as we can, but the process can feel slow at times.”
Data from successful histotripsy treatments in the #HOPEFORLIVER human trials continue to roll in. And the results, published in Radiology in September, underscore the benefits histotripsy offers in comparison to traditional treatments.
A total of 44 patients in the U.S. and Europe took part, with 42 of those cases showing successful results. That means that in 95%of the cases, tumors were destroyed without a major invasive procedure requiring lengthy recuperation, or taxing treatments such as radiation or chemotherapy.
“We feel fortunate to have treated the most patients in the trial since the technology was discovered at our university,” said Mishal Mendiratta-Lala, M.D., clinical professor of radiology at U-M and lead principal investigator of the trial, in September. “These results are very encouraging and our multidisciplinary approach will hopefully continue to improve the care of liver cancer patients.”
Of the 44 patients, major complications were reported in only three cases, but at least two were thought to be cancer-related, and not related to issues with the Edison system.
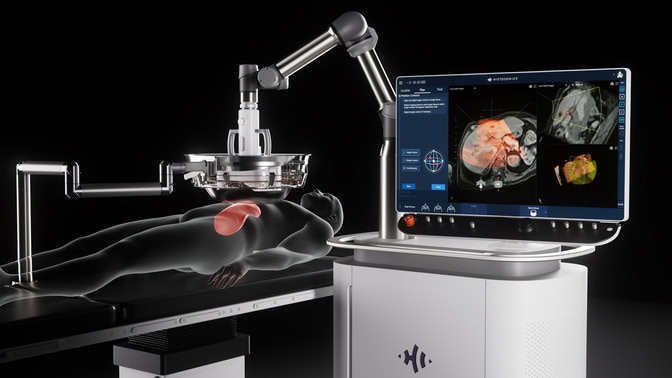
“Now that it’s being used in clinics, we’re seeing how it can treat liver tumors without damaging local major normal blood vessels, nerves and bile ducts,” Xu said. “The real-time image guidance it offers makes it highly precise and, so far, what we’re seeing is patients are experiencing no pain and fast recoveries.”
Patients continue to enroll in the #HOPE4KIDNEY trial, where histotripsy’s effectiveness in treating kidney tumors is being evaluated.
In June, the United Network for Organ Sharing listed histotripsy as an acceptable treatment option for liver cancer patients who are seeking to improve their condition enough to be eligible for a transplant.
A new human trial for treating pancreatic cancer begins this month in Barcelona, Spain. Xu and HistoSonics officials hope that a similar trial for prostate cancer could begin somewhere before the end of 2025.
Xu’s latest research focuses on how histotripsy can be used to treat sarcomas—a rare type of cancer that forms in bones and soft tissues. She hopes that sarcomas will be next in line for clinical trials.
Histotripsy uses a transducer, which converts energy into sound, to deliver pulsing ultrasound waves to a malignant mass at a precise location. When those waves hit gases inside cancerous cells, they generate clouds of millimeter-sized bubbles that repeatedly grow and collapse. The mechanical energy created breaks up the tumor cells’ structure, turning it into a harmless liquid called acellular lysate that is reabsorbed by the body.
HistoSonics has combined all of that technology into Edison, and has been the driving force behind its commercialization. And after years spent pursuing FDA approval, its arrival in October 2023 kicked things into high gear.
“Overnight, quite literally, we went from a growth stage company to a hyper growth company,” recalled Mike Blue, HistoSonics’ president and CEO. “And that’s rare. Generally, if you look at the adoption of high-end capital equipment, robotics equipment, you’ll sell a couple of systems your first year and then, maybe, a dozen systems the second year and a couple dozen the third.
“We had an order the very first day we got the FDA clearance. And since then, we’ve had over 60 orders of the Edison robotic system. That’s unheard of.”
Three months later, in early January 2024, HistoSonics announced that the first patient had received kidney treatment with the Edison device in the #HOPEFORKIDNEY trial.
In April, the Edison System was recognized with, perhaps not shockingly, an Edison gold medal award in robotic and interventional technologies. Edison awards honor “excellence in new product and service development, marketing, design and innovations.
In August, HistoSonics closed its Series D financing round with $102 million, the Wall Street Journal reported. Investor interest continues to grow, according to Jim Adox, a U-M alum, member of the Michigan Engineering Leadership Advisory Board and chairman of HistoSonics’ board of directors.
He pointed to Xu’s and HistoSonics officials’ recent trip to Hong Kong. They demonstrated an Edison device at the Chinese University of Hong Kong and participated in a “fireside chat” with Solina Chau, the Li Ka Shing Foundation’s director, at the UBS Disruptive Technology CEO Summit. The visit also led to Xu’s endowed professorship.

“It’s a huge testament of the power of this treatment,” he said. “We’re seeing passionate, philanthropic billionaires who are interested in accelerating the availability of histotripsy in their countries to treat their populations.
“We’re really just getting going.”
Today there are 30 Edison devices in use at medical centers around the U.S. and beyond.
One of those U.S. machines arrived at University of Michigan Health-West in Grand Rapids this fall, making it the second in the state following the lead of Michigan Medicine in Ann Arbor.
In its early years, HistoSonics received support from the U-M Coulter Translational Research Partnership Program, which specializes in helping researchers develop and commercialize healthcare products. The program provided consulting help and $300,000 for early testing.
Later, U-M’s Innovation Partnerships guided the research team through applying for patents and launching HistoSonics.
Those roots at U-M gave rise to what you might call a branch in 2024. The company, which has its headquarters in Minneapolis, opened a 30,000-square-foot research and development center on Ann Arbor’s south side in October. Among those on-hand to celebrate the grand opening was U-M President Santa Ono.
“This is what “Growing Michigan Together” is all about,” Ono wrote on X. “Starting blockbuster companies and ensuring that they stay in Michigan.”
The technology itself, however, has now moved far beyond Michigan—just as Xu and others had always hoped. As each Edison System reaches a new home, medical officials are touting the new services they can offer to patients. And they are not shy about what histotripsy offers.
In May, after the first treatments at the Northeast Georgia Medical Center in Gainesville, surgeon Nelson Royall told the Atlanta Journal-Constitution that histotripsy was “redefining what’s possible.”
“The gravity of this achievement,” he said, “lies in its potential to expand the boundaries of what was once thought possible in cancer treatment.”