Nanostructures get complex with electron equivalents
Nanoparticles of two different sizes break away from symmetrical designs.

Nanoparticles of two different sizes break away from symmetrical designs.
Complex crystals that mimic metals—including a structure for which there is no natural equivalent—can be achieved with a new approach to guiding nanoparticle self-assembly.
Rather than just nanoparticles that serve as “atom equivalents,” the crystals produced and interpreted by Northwestern University, University of Michigan and Argonne National Laboratory rely on even smaller particles that simulate electrons.
“We’ve learned something fundamental about the system for making new materials,” said Northwestern’s Chad Mirkin, the George B. Rathmann Professor of Chemistry in the Weinberg College of Arts and Sciences and a co-corresponding author of the paper in Nature Materials. “This strategy for breaking symmetry rewrites the rules for material design and synthesis.”
Nanoparticles have the potential to enable new materials with properties that can be carefully designed, but one of the big challenges is making these materials self-assemble. Nanoparticles are too small and numerous to build brick by brick.
Colloidal crystals are a family of self-assembled arrays made by nanoparticles, with potential applications in photonics. Crystals that can transform light may be engineered for everything from light sensors and lasers to communications and computing.
“Using large and small nanoparticles, where the smaller ones move around like electrons in a crystal of metal atoms, is a whole new approach to building complex colloidal crystal structures,” said Sharon Glotzer, the Anthony C. Lembke Department Chair of Chemical Engineering at U-M and a co-corresponding author.
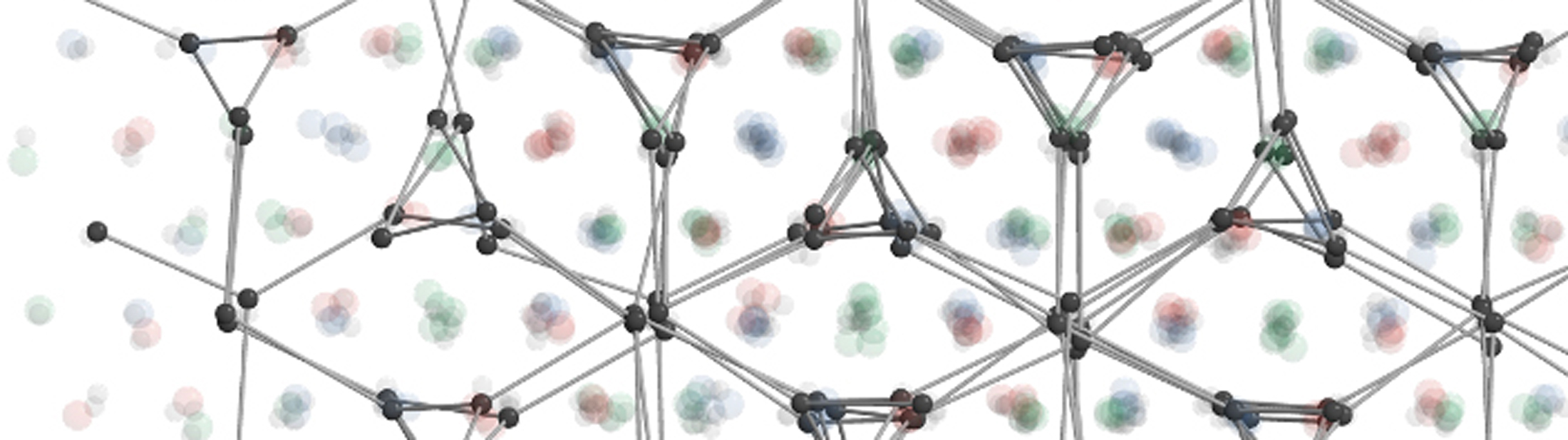
Mirkin’s team created colloidal crystals by coating metal nanoparticles with DNA to make them stick to one another. The DNA strands are self-complementary, which means they bond to one another. By tuning parameters like the length of the DNA and how densely the nanoparticles are coated, the metal nanoparticles can be “programmed” to arrange themselves in specified ways. As a result, they are called programmable atom equivalents.
However, the “atoms” in this crystal—spheres with an even coating of DNA—are the same in all directions, so they tend to build symmetric structures. To build less symmetric structures, they needed something to break the symmetry.
“Building on Chad’s prior discovery of ‘electron equivalents’ with Northwestern’s Monica Olvera De La Cruz, we explored more complex structures where control over the number of neighbors around each particle produced further symmetry-breaking,” Glotzer said.
Smaller metal spheres, with fewer DNA strands to make them less sticky, end up acting like electrons in an arrangement of larger nanoparticle “atoms.” They roved around the interior of the structure, stabilizing the lattice of large nanoparticles. Mirkin’s team varied the stickiness of the “electron” nanoparticles to get different structures, as well as changing the temperature and the ratio of nanoparticle “atoms” and “electrons.”
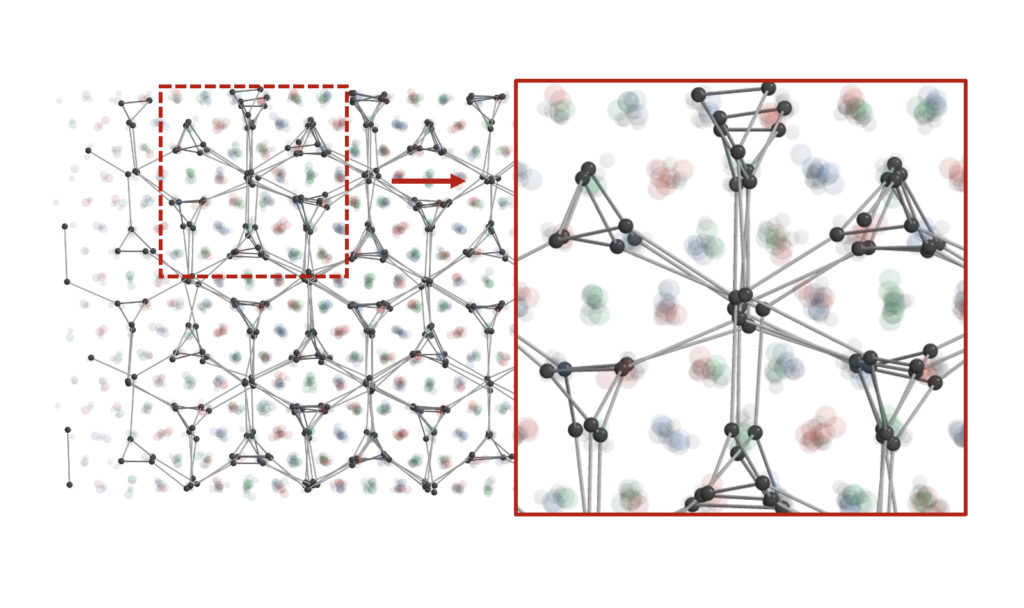
They analyzed these structures aided by small-angle x-ray scattering studies carried out with Byeongdu Lee, a physicist at Argonne National Laboratory and a co-corresponding author. That data revealed three complex, low-symmetry structures. One, whose twisted tunnels are known as a triple double-gyroid structure, has no known natural equivalent.
These new, low-symmetry colloidal crystals offer optical and catalytic properties that can’t be achieved with other crystals, and the symmetry-breaking method promises many more new structures. Glotzer’s team developed computer simulations to recreate the self-assembly results, helping to decipher the complicated patterns and revealing the mechanisms that enabled the nanoparticles to create them.
“We’re in the midst of an unprecedented era for materials discovery,” Mirkin said. “This is another step forward in bringing new, unexplored materials out of the sketchbook and into applications that can harness their incredible properties.”
The study was supported primarily by the Center for Bio-Inspired Energy Science, an Energy Frontier Research Center funded by the U.S. Department of Energy and also by the Air Force Office of Scientific Research and the Sherman Fairchild Foundation.
Mirkin is also a professor of chemical and biological engineering, biomedical engineering, and materials science and engineering at the McCormick School of Engineering; and a professor of medicine at the Feinberg School of Medicine. He also is the founding director of the International Institute for Nanotechnology. Glotzer is also the John Werner Cahn Distinguished University Professor of Engineering, the Stuart W. Churchill Collegiate Professor of Chemical Engineering, and a professor of material science and engineering, macromolecular science and engineering, and physics at U-M.