New blue fluorophore breaks efficiency records in both solids and solutions
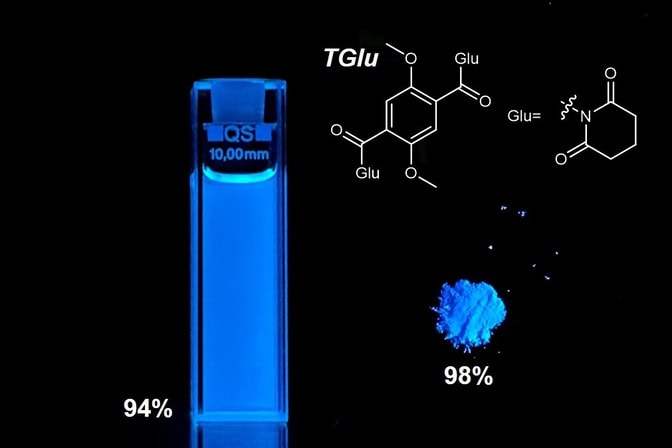
Reaching 98% efficiency in a solid state and 94% in solution, the small fluorescent molecule’s design could cut down development time and cost for future applications.

Reaching 98% efficiency in a solid state and 94% in solution, the small fluorescent molecule’s design could cut down development time and cost for future applications.
Experts

Raoul Kopelman Collegiate Professor of Science and Engineering at the University of Michigan’s Department of Materials Science and Engineering
Postdoctoral research fellow of materials science and engineering.
A new blue fluorescent molecule set new top emission efficiencies in both solid and liquid states, according to a University of Michigan-led study that could pave the way for applications in technology and medicine.
Able to absorb light and emit it at lower energy levels, fluorescent molecules called fluorophores glow in OLED displays and help doctors and scientists figure out what’s happening in cells and tissues. They need to be solid in displays and many sensing applications, but liquids are typically preferred for biological uses. Most fluorophores don’t work well in both forms, but this one does.
“The fluorescent material reached record-breaking brightness and efficiency with 98% quantum efficiency in the solid state and 94% in solution,” said Jinsang Kim, the Raoul Kopelman Collegiate Professor of Science and Engineering in the U-M Department of Materials Science and Engineering who led the study, which is published in Nature Communications.
Often, engineers designing fluorophores start in solution, exploring the optical properties of individual molecules, but run into problems in their solid-state applications when fluorophore molecules contact each other.
“Fluorophores behave very differently in the solid state, which then requires more rational molecular engineering effort for structural modification,” Kim said “By investigating and establishing a molecular design principle to make fluorophores that are bright both in solution and solid states, we have reduced development time and cost for various future applications.”
The initial discovery of the versatile fluorophore—called TGlu for short—was unexpected for lead author Jung-Moo Heo, U-M postdoctoral research fellow of materials science and engineering.

“TGlu was an intermediate step for another chemical design, but during purification I found it was surprisingly highly emissive, not only in solution but also in solid state,” Heo said.
The discovery led to the systematic study to establish the optimal design. The result was a simple design: a single benzene ring core—six carbon atoms joined in a hexagon. The researchers positioned two groups that give away electrons, called donor groups, across the ring from one another. Next to the donors, they placed two acceptor groups, which withdraw electrons, across the ring from one another.
“This so-called quadrupolar structure symmetrically distributes charge across the molecule, providing stable emission in various environments,” Heo said.
Because the ring has only six points, donor and acceptor groups are positioned next to each other. This spatial arrangement reduces the energy gap compared to other similar molecules within a compact framework, which means the fluorophore needs a relatively small amount of energy to move an electron from the ground state to an excited state—similar to jumping up a rung on a ladder.
However, the molecule’s small size means overall conjugation length remains limited—meaning electrons cannot spread out too far across the molecule. This keeps the absolute energy gap—the distance between ladder rungs—wide enough to emit blue light instead of shifting towards narrower energy gap colors like red.
Typically, small band gaps come with an efficiency drawback. When in the excited state on the higher rung of the ladder, an electron can either emit light as it comes back down to the ground state or lose energy as heat through vibration. Often, small band gaps mean more heat loss, reducing the quantum yield—an efficiency metric expressed as the percentage of absorbed UV light that gets reemitted as visible light relative to the amount lost as heat.
After trying a series of acceptor groups, the researchers found one that stabilizes the excited state. Even with the small band gap, this acceptor group prevents heat loss by restricting access to what are known as conical intersections, which function as “exit doors” for energy leakage. This unexpected behavior, called an Inverted Energy Gap Law, was confirmed both by experiments and quantum chemical simulations.
In the solid state, the acceptor groups, which were intentionally designed to be bulky, prevent the molecules from getting too close to one another which causes fluorophores to lose brightness as energy escapes as heat instead of light, a phenomenon known as quenching.
The small, highly-efficient fluorophore is simple to produce—only requiring three steps—which increases its scalability while reducing production costs.
The current TGlu design fluoresces blue light. As next steps, the researchers will adjust the band gap, and thus the color. Further, while a high quantum yield from light excitation is promising, device performance under electrical excitation requires separate testing due to additional loss mechanisms. Heo also plans to work toward a phosphorescent version of the molecule, as phosphors are overall more energy-efficient than fluorophores, for use in display technology.
Autonomous University of Madrid, University of Valencia, Eberhard Karls University Tübingen and Seoul National University also contributed to this research.