Burned rice hulls could help batteries store more charge
New research finds hard carbon in rice hull ash, providing a cheap, domestic source of the material that can replace graphite in lithium-ion or sodium-ion battery anodes.

New research finds hard carbon in rice hull ash, providing a cheap, domestic source of the material that can replace graphite in lithium-ion or sodium-ion battery anodes.
Experts

Professor of Materials Science and Engineering
Professor of Macromolecular Science and Engineering
A closer inspection of ash from burned rice hulls, the hard outer layer of rice grains, revealed a form of carbon that could nearly double the energy density of typical lithium-ion or sodium-ion batteries.
This sustainable source of ‘hard’ carbon, which outperforms ordinary graphite in battery electrodes, was discovered at the University of Michigan.
This is the first demonstration of hard carbon made through combustion. It was previously thought hard carbon could only be made by heating biomass, such as agricultural waste, to about 1200°C (2200°F) in an oxygen-free environment like nitrogen or argon.
Rather than importing graphite mined from China or Mexico, rice hull ash could provide a higher quality domestic material for making battery electrodes. The process is also more sustainable than producing graphite from biomass, which must be heated to 2000°C (3600°F) or higher—producing five to 10 tons of CO2 for every ton of battery-grade graphite.
Although most rice hulls end up in landfills, burning rice hulls provides a carbon neutral source of electricity. Wadham Energy LP in the Sacramento Valley of California generates 200,000 megawatt-hours of electricity per year by burning the agricultural byproduct—enough energy to power about 22,000 homes.
“The CO2 released while burning rice hulls comes from the same CO2 the rice plant took up from the atmosphere during photosynthesis, making the electricity produced green and carbon neutral,” said Richard Laine, U-M professor of materials science and engineering and macromolecular science and engineering and corresponding author of the study recently published in Advanced Sustainable Systems.
With about 20 billion pounds of rice grown annually in the United States, there is plenty of room to scale up.
In prior work, the research team demonstrated methods to partially remove the silica in rice hull ash which contains about 90% silica and 10% carbon. That silica can be used to produce high-purity silicon used in solar cells or semiconductors. Once the silica is partially removed from the rice hull ash through a process called depolymerization, the remaining ash is about 60%-70% carbon.
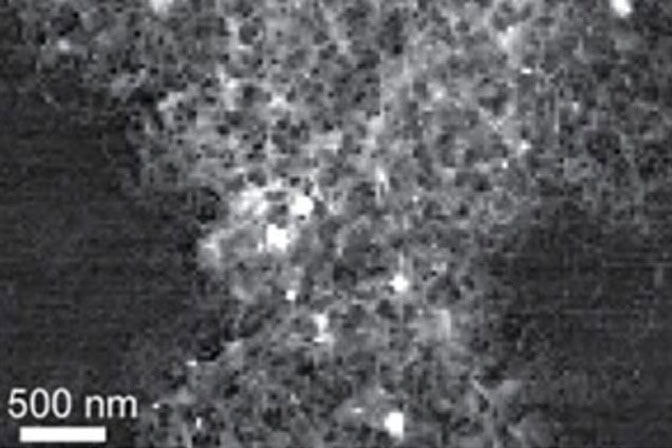
The leftover carbon was thought to be shapeless and disorganized, a material called amorphous carbon, based on the patterns made by X-rays shone through the material. However, spectroscopy techniques specialized for molecular-level detail revealed tiny islands of graphite that exist on the nanoscale (for scale, one nanometer is one billionth of a meter) within the amorphous carbon matrix. This blend of amorphous carbon dotted with graphite is called hard carbon.
“Hard carbon can be produced by combustion in this case because as you burn away the carbon of rice hulls, you create a shell of silica around the remaining carbon and it bakes it like a pie,” Laine said.

When testing the electrochemical properties of hard carbon obtained from rice hull ash, it outperformed both commercial hard carbon and graphite as the anode of a lithium-ion battery, the point where charge flows out of the battery.
A gram of commercial hard carbon accepts enough lithium to store about 500 milliampere-hours (mAh)—a unit of electrical charge often used to describe battery storage capacity. In contrast, a gram of graphite accepts about 370 mAh, meaning hard carbon batteries have about 50% higher energy density. Rice hull ash hard carbon exceeds both, with a storage capacity of more than 700 mAh—nearly double that of graphite.
The nanoporous structure of the isolated hard carbon is thought to contribute to the increased lithium capacity.
Turning agricultural waste into a valuable product, rice hull ash hard carbon can help meet the growing demand for batteries for use in electric vehicles and storing intermittent renewable energy while decreasing both cost and emissions.
The team has applied for patent protection with the assistance of U-M Innovation Partnerships and is seeking partners to bring the technology to market.
Karlsruhe Institute of Technology in Germany also participated in this research through co-author Sylvio Indris. Wadham Energy supplied the rice hull ash used in the research.
The process was studied in part at the Michigan Center for Materials Characterization. The work at the University of Michigan was primarily funded by the National Science Foundation and the Mercedes-Benz Research & Development North America.