
Squishy, metal-free magnets to power robots and guide medical implants
Strong enough to move soft robots and medical capsules, weak enough to not ruin MRI images.

Strong enough to move soft robots and medical capsules, weak enough to not ruin MRI images.
Experts
“Soft robots,” medical devices and implants, and next-generation drug delivery methods could soon be guided with magnetism—thanks to a metal-free magnetic gel developed by researchers at the University of Michigan and the Max Planck Institute for Intelligent Systems in Stuttgart, Germany.
The material is the first in which carbon-based, magnetic molecules are chemically bonded to the molecular network of a gel, creating a flexible, long-lived magnet for soft robotics. The study describing the material was published today in the journal Matter.
Creating robots from flexible materials allows them to contort in unique ways, handle delicate objects and explore places that other robots cannot. More rigid robots would be crushed by the deep ocean’s pressure or could damage sensitive tissues in the human body, for example.
“If you make robots soft, you need to come up with new ways to give them power and make them move so that they can do work,” said Abdon Pena-Francesch, assistant professor of materials science and engineering affiliated with the Robotics Institute at the University of Michigan and a corresponding author of the study.

Today’s prototypes typically move with hydraulics or mechanical wires, which require the robot to be tethered to a power source or controller, also limiting where they can go. Magnets could unleash these robots, enabling them to be moved by magnetic fields.
Conventional, metallic magnets introduce their own complications, however. They could reduce the flexibility of soft robots and be too toxic for some medical applications.
The new gel could be a nontoxic alternative for medical operations, and further modifications to the magnet’s chemical structure could help it degrade in the environment and human body. Such biodegradable magnets could be used in capsules that are guided to targeted locations of the body to release medicine.
“If these materials can safely degrade in your body, you don’t have to retrieve them with another surgery later,” Pena-Francesch said. “This is still pretty exploratory, but these materials could enable newer, cheaper medical operations some day.”
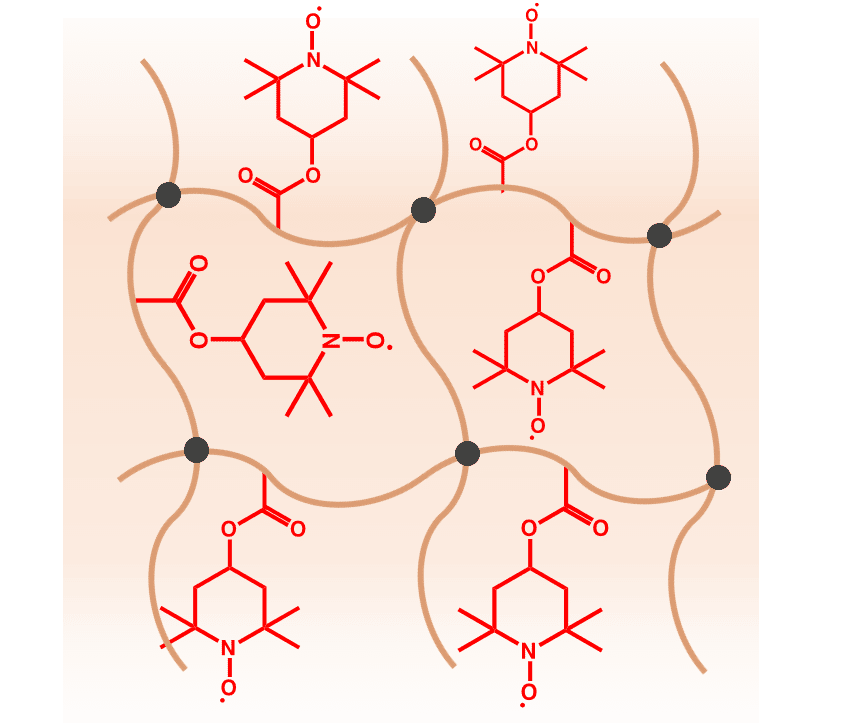
The team’s gel consists solely of carbon-based molecules. The key ingredient is TEMPO, a molecule with a “free” electron that is not paired up with another electron inside an atomic bond. The spin of every unpaired TEMPO electron in the gel aligns under a magnetic field, which attracts the gel to other magnetic materials.

Additional “cross-linking molecules” in the gel act like a frame that connects the TEMPO molecules to a solid network structure while forming a protective cage around the TEMPO electrons. That cage prevents the unpaired electrons from forming bonds, which would remove the gel’s magnetic properties.
“Earlier studies soaked these small, magnetic molecules into a gel, but they could leak out of the gel,” said Zane Zhang, a doctoral student in materials science and engineering and co-author of the study. “By integrating the magnetic molecules into the cross-linked gel network, they are fixed inside.”
Locking the TEMPO molecules inside the material ensures that the gel does not leak potentially harmful TEMPO molecules into the body and allows the material to retain its magnetic properties for more than a year.
While weaker than metallic magnets, the TEMPO magnets are strong enough to be pulled and bent with another magnet. Their weaker magnetism also has some upsides—TEMPO magnets can be photographed by an MRI, unlike stronger magnets that can distort MRI images to the point of uselessness.
“Medical devices using our magnets could be used to deliver drugs to target locations and measure tissue adhesion and mechanics in the GI tract under MRI imaging,” said Metin Sitti, former director of the Physical Intelligence Department at Max Planck Institute for Intelligent Systems and a corresponding author of the study.
The research is funded by the American Chemical Society Petroleum Research Fund, Max Planck Society and the European Research Council Advanced Grant Soft-bodied Miniature Mobile Robots program. The material was built in the BioInspired Materials Laboratory and characterized in the Michigan Center for Materials Characterization.
Pena-Francesch is also an assistant professor of chemical engineering and macromolecular science and engineering.