Durable coating kills the COVID virus and other germs in minutes
Polyurethane locks in the antimicrobial power of tea tree and cinnamon oils. The new technology could start making public spaces safer within a year.

Polyurethane locks in the antimicrobial power of tea tree and cinnamon oils. The new technology could start making public spaces safer within a year.
U-M EXPERTS:
Humans may soon have a new weapon in our centuries-old battle against germs: the first durable coating that can quickly kill bacteria and viruses and keep on killing them for months at a time.
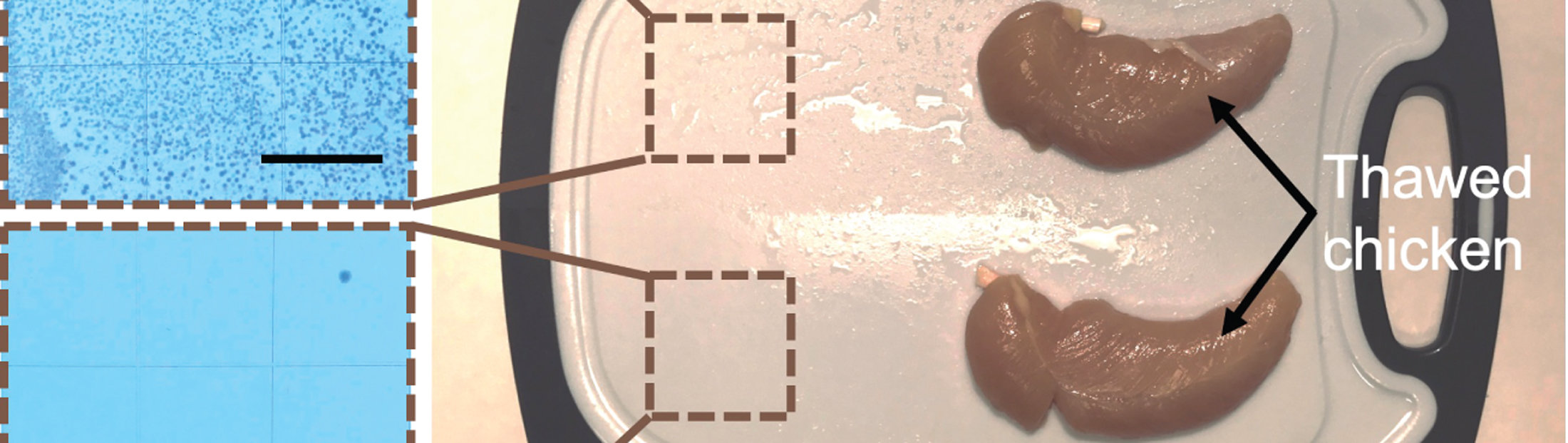
Developed by a team of University of Michigan engineers and immunologists, it proved deadly to SARS-CoV-2 (the virus that causes COVID-19), E. coli, MRSA and a variety of other pathogens in a recent study. It stood up to months of repeated cleaning, abrasion and other punishment on real-world surfaces like keyboards, cell phone screens and even chicken-slathered cutting boards.
The coating could be a game changer in traditionally germ-laden public spaces like airports and hospitals, according to Anish Tuteja, a professor of material science and engineering at U-M and co-corresponding author of the paper published in Matter.
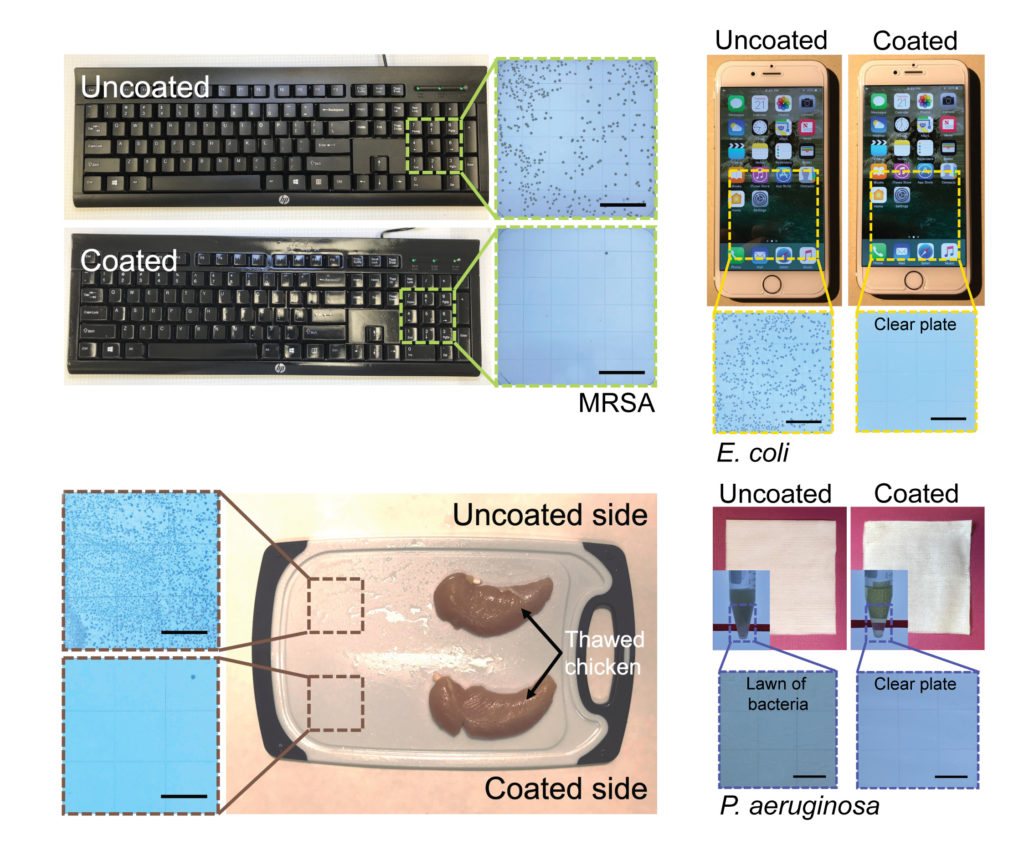
“We’ve never had a good way to keep constantly-touched surfaces like airport touch screens clean,” he said. “Disinfectant cleaners can kill germs in only a minute or two but they dissipate quickly and leave surfaces vulnerable to reinfection. We do have long-lasting antibacterial surfaces based on metals like copper and zinc, but they take hours to kill bacteria. This coating offers the best of both worlds.”
The coating, which is clear and can be brushed or sprayed on, gets its durability and germ-killing power by combining tried-and-true ingredients in a new way. It uses antimicrobial molecules derived from tea tree oil and cinnamon oil, both used for centuries as safe and effective germ killers that work in under two minutes. The coating’s durability comes from polyurethane, a tough, varnish-like sealer that’s commonly used on surfaces like floors and furniture.
“The antimicrobials we tested are classified as ‘generally regarded as safe’ by the FDA, and some have even been approved as food additives,” Tuteja said. “Polyurethane is a safe and very commonly used coating. But we did do toxicity testing just to be sure, and we found that our particular combination of ingredients is even safer than many of today’s antimicrobials.”
The results of the study’s durability tests suggest that the coating could keep killing germs for six months or longer before its oil begins to evaporate and reduce its disinfectant power. But even then, Tuteja says it can be recharged by wiping it with fresh oil; the new oil is reabsorbed by the surface, starting the cycle again.

Tuteja estimates that the technology could be commercially available within a year; it has been licensed to Hygratek, a spinoff company that Tuteja founded with assistance from the U-M Office of Technology transfer.
The key challenge was to combine the oil and polyurethane in a way that let the oil molecules do their germ-killing work while preventing them from evaporating quickly. To solve the problem, Tuteja convened a team of chemical and materials science engineers at U-M that included co-corresponding author and associate professor of materials science and engineering and biomedical engineering Geeta Mehta; materials science and engineering PhD students Abhishek Dhyani, Taylor Repetto, co-first authors, Sarah A. Snyder and Catherine Snyder; and macromolecular science and engineering master’s student Zhihe Gao.
They found a possible solution in cross-linking, a well-known process that uses heating to link materials together at the molecular level. The smaller oil molecules readily combined with the cross-linking polymer molecules, forming a stable matrix.
But to kill germs, the oil molecules need to penetrate their cell walls, which they can’t do if they’re tightly tethered into the matrix. Eventually, they found a middle ground by partially cross-linking the materials—enough to keep some of the oil molecules free to do their work, but keeping others bound tightly to the polyurethane.
“There was some trial and error, but we eventually found that cross-linking only some of the oil did what we needed,” Tuteja said. “Some oil does stay free, but it tends to want to stay with the oil that’s cross-linked into the matrix, and that helps the coating last longer.”
Once the basic recipe was set, the researchers set about finding a combination of active ingredients that would kill a wide variety of the germs that trouble humans most. They worked with co-corresponding authors Christiane E. Wobus, an associate professor of microbiology and immunology and J. Scott VanEpps, an assistant professor of emergency medicine, both at the U-M Medical School, as well as immunology PhD students Dylan Bartikofsky and Carmen Mirabelli, to test the coating against a wide variety of viruses and bacteria.
“There are many factors that go into deciding which pathogens to target,” VanEpps said. “The germs you’re likely to encounter in a kitchen, for example, are much different from those in a hospital. Also, you ideally want to control pathogens without eradicating the beneficial bacteria that we humans live in a normal, happy relationship with.”
Test subjects included SARS-CoV-2, E. coli, the MRSA bacteria that can cause serious hospital infections and many others. Ultimately, they found a precise balance of antimicrobial molecules that were effective, safe and inexpensive.
Tuteja also emphasizes that they’re not locked into one specific formula. While the recipe in the study is a good balance of germ-killing power and durability, the team’s understanding of the basic properties of individual ingredients enables them to tweak the formula for specific applications—for example, to optimize the balance between disinfectant strength and durability or to rebalance the combination of antimicrobial agents to kill specific germs.
“It’s never our goal just to develop a one-off coating, but instead to develop a library of underlying material properties to draw from,” Tuteja said. “If we can understand those properties, then we can develop any number of coatings to meet the needs of specific applications.”
The study was funded by the Office of Naval Research, with additional support from the University of Michigan, Marie Skłodowska-Curie Actions, the National Institutes of Health and the Department of Defense, with raw materials provided by Covestro.
The University of Michigan has applied for a patent based on this technology. Tuteja and the University of Michigan have a financial interest in Hygratek.