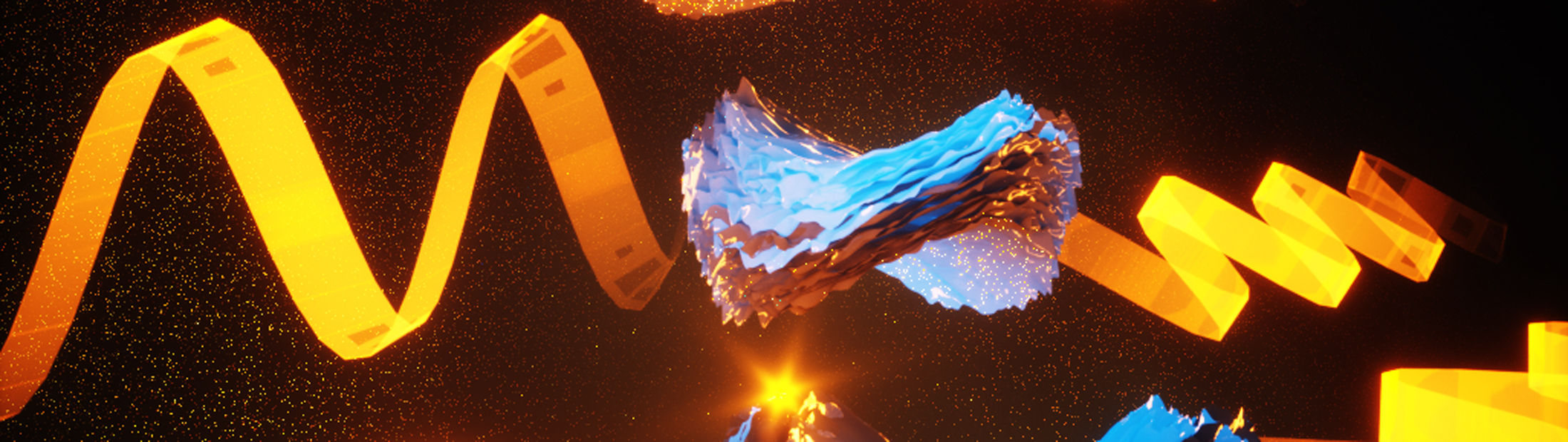
For the first time, controlling the degree of twist in nanostructure particles
Being able to decide not only whether a micron-scale particle twists but also how much could open new avenues for machine vision and more.

Being able to decide not only whether a micron-scale particle twists but also how much could open new avenues for machine vision and more.
Micron-sized “bowties,” self-assembled from nanoparticles, form a variety of different curling shapes that can be precisely controlled, a research team led by the University of Michigan has shown.
The development opens the way for easily producing materials that interact with twisted light, providing new tools for machine vision and producing medicines.
While biology is full of twisted structures like DNA, known as chiral structures, the degree of twist is locked in—trying to change it breaks the structure. Now, researchers can engineer the degree of twist.
Such materials could enable robots to accurately navigate complex human environments. Twisted structures would encode information in the shapes of the light waves that reflect from the surface, rather than in the 2D arrangement of symbols that comprises most human-read signs. This would take advantage of an aspect of light that humans can barely sense, known as polarization. The twisted nanostructures preferentially reflect certain kinds of circularly polarized light, a shape that twists as it moves through space.
“It is basically like polarization vision in crustaceans,” said Nicholas Kotov, the Irving Langmuir Distinguished University Professor of Chemical Sciences and Engineering, who led the study published on the cover of Nature. “They pick up a lot of information in spite of murky environments.”
Robots could read signs that look like white dots to human eyes; the information would be encoded in the combination of frequencies reflected, the tightness of the twist and whether the twist was left- or right-handed.
By avoiding the use of natural and ambient light, relying instead on circularly polarized light generated by the robot, robots are less likely to miss or misinterpret a cue, whether in bright or dark environments. Materials that can selectively reflect twisted light, known as chiral metamaterials, are usually hard to make—but the bowties aren’t.
“Previously, chiral metasurfaces have been made with great difficulty using multimillion dollar equipment. Now, these complex surfaces with multiple attractive uses can be printed like a photograph,” Kotov said.
Twisted nanostructures could also help create the right conditions to produce the twisting molecules in chiral medicines. Human-designed reactions usually make both versions in equal numbers, but nature often produces and uses just one version, and the other can run the gamut from inactive to toxic. It would be far preferable to make only the useful version of the molecule.
The bowties are porous structures, and the researchers believe that the pores are also chiral. These pores could potentially function as tiny reaction chambers that preferentially or even exclusively produce the right- or left-handed version.
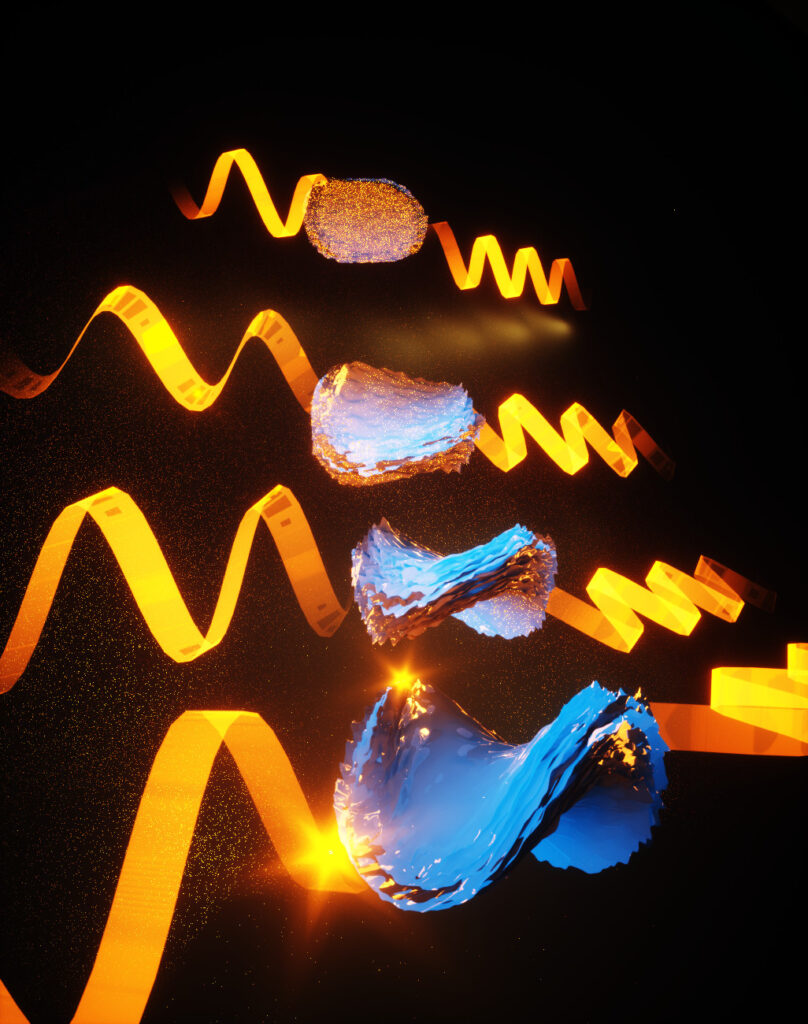
“What hasn’t been seen in any chiral systems before is that we can control the twist from a fully twisted left-handed structure to a flat pancake to a fully twisted right-handed structure. We call this a chirality continuum,” said Prashant Kumar, a post-doctoral research fellow in chemical engineering at U-M and first author of the study in Nature.
Kumar tested the bowties as a sort of paint, mixing them with polyacrylic acid and dabbing them onto glass, fabric, plastic and other materials. Experiments with lasers showed that this paint reflected twisted light only when the twist in the light matched the twist in the bowtie shape.

In making the bowties, Kumar started with a solution of water, sodium hydroxide (also known as lye), and cystine, a protein fragment that comes in left and right handed versions. To that, he added cadmium metal. First, the cadmium and cystine wove themselves into nano-plates, just 1.2 nanometers thick, or about a millionth of a millimeter, and 50 to 200 nanometers wide. Then, they grew into ribbons and finally bowtie-like shapes with a candy-wrapper twist.
If the cystine was all left-handed, they made left-handed bowties, and right-handed cystine yielded right-handed bowties. But with different ratios of left-and right-handed cystine, they made intermediate twists, including the flat pancake at a 50-50 ratio. The pitch of the tightest bowties, basically the length of a 360-degree turn, is about 4000 nanometers long, within infrared light’s range of wavelengths.
In contrast with other chiral nanostructures, which can take days to self-assemble, the bowties formed in just 90 seconds. This meant that Kumar along with two seniors in chemical engineering at U-M, Alexander Simon and Daniel Katz, could try hundreds of different reaction conditions each week. They produced 5000 different shapes within the bowtie spectrum.
Kumar, along with Wenqian Xu, a staff scientist at Argonne National Laboratory, uncovered the structure of the bowties in atomic detail using X-rays. Then, Thi Vo, who was a U-M postdoctoral researcher in chemical engineering at the time, used computer simulations to explore exactly how the bowties formed.
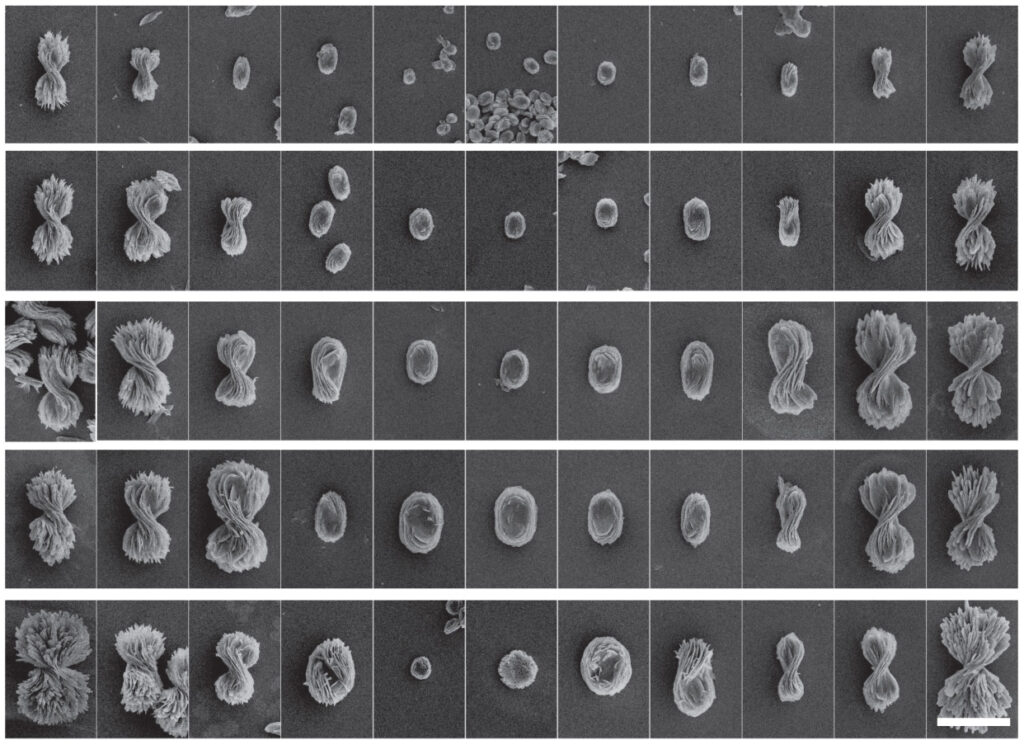
“Not only do we know the progression from the atomic scale all the way up to the micron-scale of the bowties, we also have theory and experiments that show us the guiding forces. With that fundamental understanding, you can design a bunch of other particles,” said Vo, who worked with Sharon Glotzer, co-corresponding author of the study and the Anthony C. Lembke Department Chair of Chemical Engineering at U-M.
Additional material analysis and contributions to theory were provided by collaborators at U-M, the University of Pennsylvania, the University of Palermo in Italy and Pro Vitam Ltd, Romania.
This study was supported by the Office of Naval Research, the National Science Foundation, and the Army Research Office.
Kotov is also the Joseph B. and Florence V. Cejka Professor of Engineering and a professor of chemical engineering and macromolecular science and engineering. Vo is now a professor of chemical and biomolecular engineering at Johns Hopkins University. Glotzer is also the John Werner Cahn Distinguished University Professor of Engineering, the Stuart W. Churchill Collegiate Professor of Chemical Engineering, and a professor of materials science and engineering, macromolecular science and engineering and physics. Simon and Katz are now PhD students in chemical engineering at Northwestern University and the University of California Los Angeles, respectively.