Opening the black box of human development
New methods for studying embryonic development could enable better fertility treatment and prevent congenital disabilities, but they also pose tough questions.

New methods for studying embryonic development could enable better fertility treatment and prevent congenital disabilities, but they also pose tough questions.
It is difficult to study the journey from single cell to human infant, unfolding like a miracle in the dark of the womb. But sometimes, it seems less than miraculous. We don’t know why embryo after embryo miscarried. Why this child has a cleft lip, a heart murmur, a clubfoot. Why her spine didn’t close, why his brain didn’t form. There is more pain in these mysteries than we are equipped to express in the pages of The Michigan Engineer.
But one of our engineers is a pioneer in a new field looking for explanations. Jianping Fu, an associate professor of mechanical engineering, works closely with Deborah Gumucio, the James Douglas Engel Collegiate Professor and a professor emerita of cell and developmental biology at U-M, on using stem cells to model parts of very early embryos.
They are among a few groups around the world using human stem cells to gain new insights into the crucial but essentially unobservable window when the embryo’s cells take their first steps toward organizing into a body.
“There’s a great group of images called the Carnegie embryo collection, at the National Museum of Health and Medicine, that captures human embryos from implantation all the way to the fetal stage,” said Gumucio. “But these are just individual static pictures. They don’t say anything about cell dynamics.”
And cell dynamics are critical in these early weeks, as an embryo goes from a bundle of identical cells to a miniature body with the beginnings of limbs and organs. A small mistake here can bring on a miscarriage or have life-long consequences. So researchers want to study what goes right, what goes wrong, and whether there is any way to prevent problems from arising.
Lab-grown embryos could broaden our view, but the ethics are complex. At present, these embryos must be destroyed before they reach 14 consecutive days of development, in accordance with international conventions.
But it may be that some important questions could be answered without growing whole embryos – by instead building partial, model embryos out of stem cells. One prominent ethicist argues that this field stands outside the scope of conventional bioethics and engineering ethics, requiring us to weigh risk, cost and benefit in new ways.
Because the question at the core of the problem has no good answer: When does a clump of cells become human?
Western thought has been divided on when human cells gain the “moral status” of a human being for as long as we have written records. Conception, 40 days after conception, and at the first breath are historically popular milestones.
Developmental biology and medicine have unveiled the timing of more thresholds that many find significant. The heart’s future pacemaker cells start signalling around three weeks after conception, sometimes referred to as a heartbeat even though the heart will take a few more weeks to form. The first brain activity begins at roughly six weeks (near the 40-day mark, coincidentally). The earliest surviving premature births are in the 22nd week – about halfway to full term.
Because many people embrace an ideology that defines the threshold of moral status, it’s hard to reach a compromise on the issue. And yet, in the early 1980s, that is precisely what researchers, ethicists and public servants in the United Kingdom did.
The Warnock report of 1984 recommended that researchers should be allowed to study human embryos for up to 14 days. It enabled research that explained aspects of infertility, and it continues to support the improvement of IVF treatments. The timing was chosen in part because that’s the moment when the embryo becomes a single individual – it can no longer split into identical twins. This standard was adopted around the world.
But an international standard is not a law. In the U.S., there is no federal law on embryo research. The restriction at the national level is on tax dollars: federal agencies don’t fund research that involves the creation or destruction of embryos. At the state level, it’s a patchwork. Louisiana forbids embryo research. Michigan allows it.
The 14-day rule also kicked a thorny social and philosophical problem down the road. In 1984, researchers could hardly keep embryos alive for a few days. Only in 2016 did that hedge catch up with us, when researchers first had to deliberately terminate lab-grown embryos at 13 days. But a couple years before researchers hit this milestone, others were discovering that stem cells could reproduce some of the cell patterns and structures found in early embryos.
matical and computational biology, and Ali H. Brivanlou, who heads the laboratory of stem cell biology and molecular embryology, both at Rockefeller University.
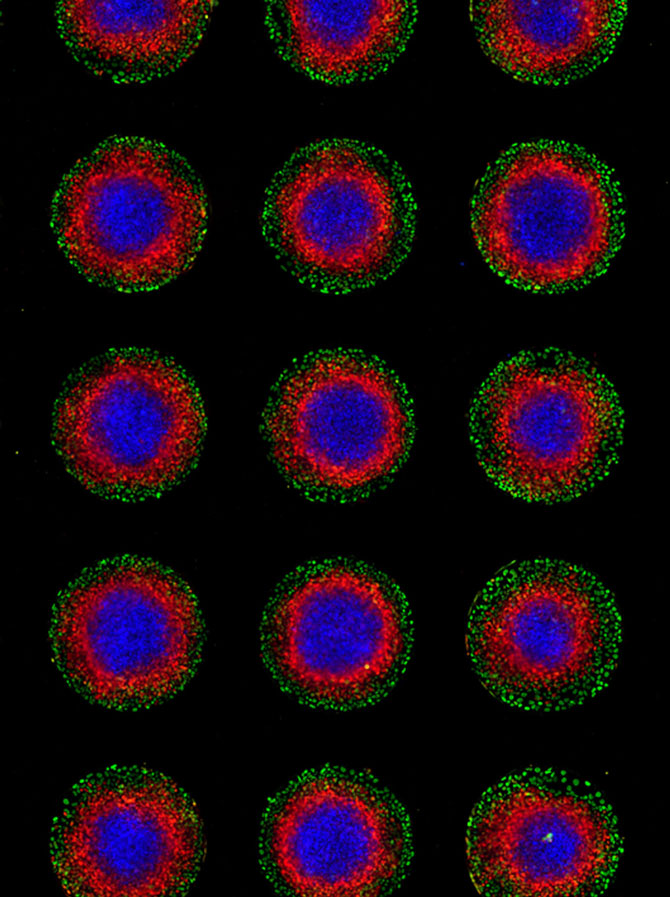
Brivanlou went on to lead one of those 13-day teams, but in his collaboration with Siggia, the group coaxed stem cells to differentiate into the three cell types that would go on to form all the organs and tissues of the body – a process called gastrulation. In a real embryo, these cell types layer one atop the other, inside a sphere of support tissues. In the model, the cells arranged themselves in concentric circles. Even so, they provided insights into gastrulation.
The three cell types are ectoderm, mesoderm and endoderm. The ectoderm primarily turns into skin and the nervous system, including the brain – in general, the outermost parts of our bodies. The endoderm mainly produces organs associated with fueling the body: the gut, the lungs and those that produce the hormones of metabolism. The mesoderm, in the middle, makes everything else – including muscle, bone, blood, the heart, and reproductive organs.
In the cell cultures, the support cells formed the outer ring, then endoderm, mesoderm and, in the center, ectoderm. The team undertook this experiment because they were interested in cell signaling. How do cells talk to each other in order to organize themselves into an embryo?
“There’s a sort of black hole in our knowledge of human embryos, from implantation to about four weeks after conception,” said Siggia.
They set the stage with micropatterned plates, which encouraged the cells to form a colony with a radius and density similar to that of an epiblast: the set of stem cells that eventually become the fetus.
Then, they exposed the cells to a growth factor called bone morphogenic protein (BMP), which is known to trigger gastrulation in mammals. The cells took it from there. First, the cells on the outside started differentiating and becoming support tissues that would go on to form the placenta, among other things. They began sending out their own signals, known as Wnt and Nodal. These signals can be thought of as very slow waves washing over the cells.
Aryeh Warmflash, formerly a postdoctoral researcher under Siggia and Brivanlou and now an assistant professor of biosciences at Rice University, has used this platform to continue exploring how chemical signals operate. One of his recent studies demonstrated that the cells aren’t just paying attention to the height of Wnt and Nodal waves: they also notice the time that passes in between their arrivals.
“If we think about a cell sitting somewhere and a cell sitting 100 microns away, they actually see the same profile of signaling, it’s just displaced in time. So what happens for one cell will happen for the other cell hours later,” said Warmflash.
In this way, cells figure out how far they are from the edge of the colony and whether they should be endoderm or mesoderm. The waves run out before they reach the center of the circle, so those cells become ectoderm.
Now, Warmflash is trying to work out how to get this happening in 3D rather than 2D: three vertical layers encircled by a fourth, rather than four concentric circles. He’s also connecting with clinicians to begin exploring how developmental problems arise using stem cells derived from patients.
Most of us won’t look at a bulls-eye-shaped cell colony and see human potential, but a professor of bioethics at Case Western Reserve and now Harvard too saw where this was going. In 2015, Insoo Hyun began writing about what gastrulating stem cells could mean for ethics.
He researched bioethics and engineering ethics, eventually settling on the need for a new paradigm: bioengineering ethics. Because this isn’t just about what you can do with a living research subject or the negative impacts a technology may have on people. It’s whether it is ethical to engineer a biological system that would normally be out of bounds experimentally – if it enables you to answer important questions.
He wasn’t alone. Researchers at Harvard Medical School wrote a paper on “synthetic human entities with embryo-like features,” or SHEEFs. It was an early deep dive into what these embryo-like structures – no one yet agrees on a name – mean for the 14-day rule.
The 14-day rule treats human development like a road from fertilized egg to baby that can be blocked off well before those cells are widely believed to have achieved moral status. But existing stem cell protocols already allow researchers to build structures beyond that roadblock. For instance, Siggia pointed out that stem cell models of fetal brains have already been developed and utilized to study how the Zika virus harms brain development.
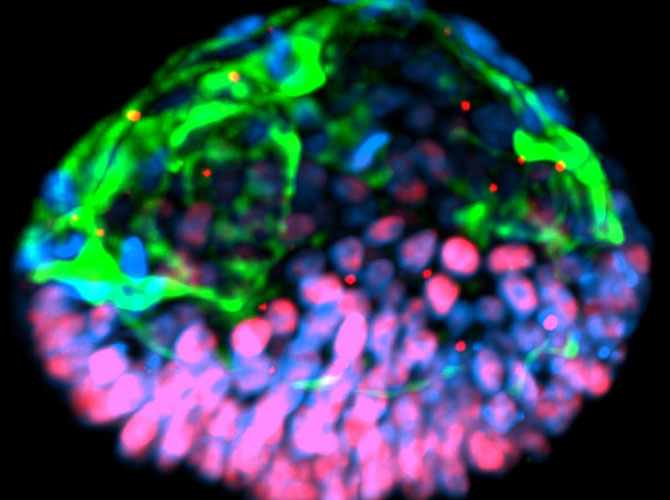
Shortly after the publication of the SHEEF paper, Fu and Gumucio made a splash in the press with their own work at U-M. They published a paper reporting a structure generated from stem cells that resembled part of a human embryo – an amniotic sac with an attached epiblast. MIT Technology Review ran an article called, “Artificial embryos are coming, and no one knows how to handle them.”
Fu and Gumucio were surprised by the headline. Their models represent only portions of particular moments in embryonic development, rather than being actual synthetic embryos. They arose as a side effect of trying to produce amnion, or the beginnings of the amniotic sac that protects the developing fetus. Until their 2016 paper, no one had ever reported making amnion from stem cells.
They had suspected that the chemical signals attempted before were not enough – the stem cells in the embryo needed a mechanical signal to know that they had implanted in the uterus, and it was time to make amnion. So, Fu’s group made a mechanical model of the uterus: a thick, soft bed of gel for the uterine wall, covered with a layer of looser gel for the uterine lining.
They achieved their goal – for the first time, they saw stem cells turning into amnion. But there was more. Sometimes, in addition to creating the ball of amnion, a set of stem cells retained their potential, lining up just like cells in an epiblast.
“Before making amnion, we never thought we could obtain these embryo-like structures,” said Fu. “That’s the beauty of life science, right? Stem cells have these amazing self-organizing properties. As an engineer, we just generate controllable culture systems that provide the right instructions for the cells.”
They even observed cells differentiating in a way that resembles the posterior end of the epiblast – the part that goes on to form the lower portion of the fetus. Some interpret this as a rough representation of the “primitive streak,” a line that forms on the surface of the epiblast. It is the first indication of which end will be the head and which will be the rear.
While it started out as a fluke, the team pursued this development, trying to understand why it happened. And, as Warmflash and colleagues before them had found, it turned out that the size of the stem cell colony they started with was critical. They also found that they didn’t need the most controversial stem cells, derived directly from embryos. Rather, reprogrammed adult cells could also form these synthetic amnion-epiblast structures.
In July 2019, Siggia and Brivanlou – the scientists behind the concentric circle embryonic models – came out with a new 3D model focused on the epiblast. The epiblast is normally a disc, also known as the embryonic disc, but their new version is a spherical shell. Like Fu and Gumucio, they’ve seen signs of the primitive streak, although in their shell it comes out as a sector rather than a line.
Still, Siggia’s team was able to see how some of the early signaling operates. They induced the cells in their shells to start differentiating with a dose of BMP, which caused the cells to begin producing Wnt, as in their earlier experiments. Where the primitive streak formed, the cells began producing a signal that inhibits Wnt. This might be part of the signal that tells the primitive streak to stop its march across the epiblast, defining the future head and rear ends of the body.
Until a recent mouse embryo experiment, reported in 2016, the epiblast wasn’t thought to make a Wnt-inhibiting signal – that was believed to come from support cells. But even with this result from Siggia’s team, we can’t be certain that this is the way a real human embryo behaves.
That is one of the challenges of using stem cells to model human embryos. We can see how well the stem cell structures align with the limited microscope images we have of real embryos. What we can’t see is how well the stem cells approximate cell signaling in real embryos – for that, we’d need embryo experiments beyond 14 days.
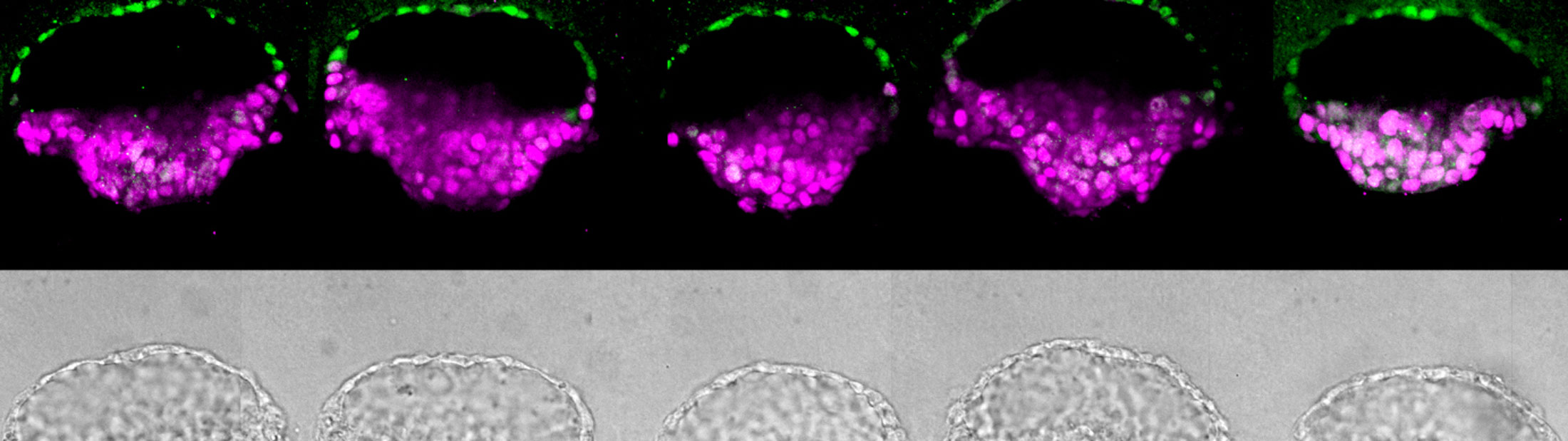
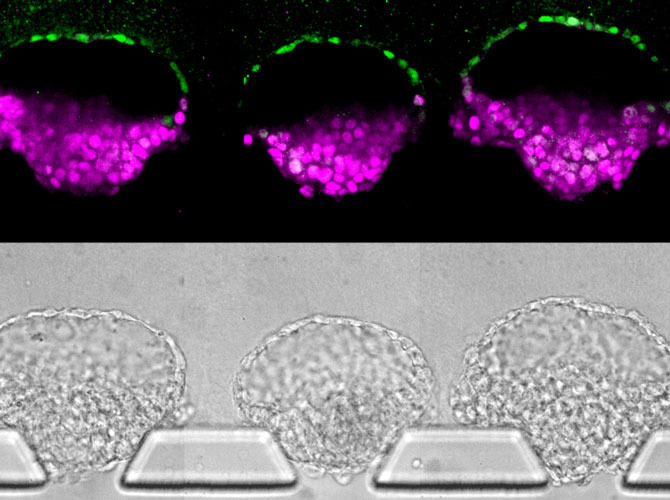
Meanwhile, Fu had taken the implantation concept and done what engineers do: made the process very reproducible and scalable. Now, as reported in a paper published in the journal Nature, Fu can make these essential pieces of the embryo in batches.
“In conventional 3D cultures without external controls of what stem cells do, less than 5 percent of the stem cell clusters formed embryo-like structures,” said Fu. “With our microfluidic system, which gives us a handle to precisely control the culture environment, we can achieve a superior efficiency of above 90% for generating these embryo-like structures.”
This controllability, reproducibility and scalability could enable the screening of medicines and chemicals for safety during pregnancy [see The case for embryoids].
“I really do like their approach because they are trying to model aspects of the natural human embryo in their synthetic work but not trying to model every aspect,” said Hyun. “If you can still answer your research questions by modeling the system of interest without recreating every aspect of the natural embryo, then that’s probably a prudent way forward.”
Their new method relies on three tiny channels in a microfluidic device. The center channel, filled with gel that serves as a model uterine wall, is flanked by one channel that delivers stem cells and another that delivers chemical signals. Where there are breaks in the walls between the channels, the gel naturally forms little wells that capture stem cells.
The cells grow into colonies shaped like hollow balls which then burrow into the gel. This mechanical signal, with help from the chemical signals in the device, helped turn some of the stem cells into amnion cells. This produced the amnion-epiblast models that the team had seen before. And as before, the epiblast cells began to differentiate in a way that roughly resembled the posterior end.
But toward the end of the experiment, they saw a different kind of cell popping up in both the amnion and the epiblast parts. From their gene expression, they looked like primordial germ cells – cells that go on to make eggs and sperm, and which are expected to show up at about this time.
They also found that they could block Wnt, and the posterior-like differentiation wouldn’t occur. This meant that the epiblast-like portion was on track to represent the head end of the embryonic disk.
“This method could help researchers to advance our understanding of early human development and develop new diagnostic tools for fertility problems,” said Gumucio.
Even though their stem cell models are a far cry from true embryos, Fu and his research team have carefully limited their experiments, ending them within five days after the cells began to self-organize. Still, during the vetting process for their most recent paper, one of the scientists who reviewed it asked whether they could incorporate missing support tissues into their models. In other words, could they make a synthetic embryo that might have the potential to grow into a human?
Fu was taken aback that anyone would suggest they should have tried this. Mouse cells, however, have already started down this road.
Teams at the University of Cambridge in the UK and in the Netherlands had independently generated whole synthetic mouse embryos by mixing the precursor cells from mouse fetus and extraembryonic tissues. They were good enough facsimiles that they actually implanted in the wombs of mice, though they didn’t grow into mouse pups. While no one has yet made precursor cells of the human placenta and yolk sac, we should decide on ethical boundaries before this capability exists.
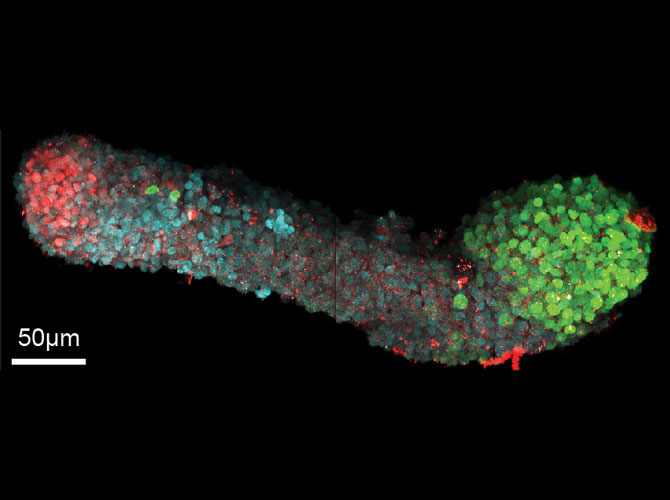
What they do have is all the right symmetries. The head is different from the rear, the belly is different from the back, and the right and left are mirror images of one another.
And the individual cells are transforming as expected: differentiating into ectoderm, mesoderm and endoderm. Then, they go further, becoming recognizable organ-like cells—even though there aren’t clear boundaries between tissues. Martinez-Arias sees potential to better understand the chemical signals that tell cells what to become and when.
Like other embryoids, the gastruloids aren’t blocking Wnt, so they aren’t on a path toward a brain. As for ethics, Martinez-Arias sees his embryoids as merely a branch of another field: organoids.
Carefully controlling the size of a stem cell culture to push cells toward embryo-like behaviors works even beyond the 14-day mark, as Alfonso Martinez-Arias, a professor of genetics at the University of Cambridge, and his team have found. Although his team’s experiments have so far focused on mouse stem cells, their approach represents a direction that human cell research could take.
“The question that many of us have is how is an organism built,” said Martinez-Arias. He explained that this had previously been explored through genetics: knocking out one gene at a time to figure out what it did.
But over the course of his career he realized, “Building an organism is the business of cells, not so much the business of genes.”
So he began to look instead at how cells decide what to do, particularly in early development. His group’s method collects clusters of stem cells in conventional microwell plates – a plastic plate full of round divots that hold individual cell colonies. If those cells are in colonies the size of an epiblast, they’ll begin gastrulation when they get a dose of Wnt.
Martinez-Arias’s structures, called gastruloids, aren’t exactly a high-fidelity rendition of a developing embryo. While real mouse embryos have definitive structures by the 8-day point, the gastruloids look more like a cloud of cells in a general embryo-like shape.

Perhaps the core of the abortion debate is whether an embryo is a part of a woman’s body or its own entity. Answering to the orders of its own genes while also being totally dependent on its mother’s body, it seems to be neither and both.
From the viewpoint of the laboratory, however, growing a synthetic embryo is not so different from growing a synthetic kidney. Jason Spence, a professor of cell and developmental biology at U-M and an internationally recognized researcher in organoids, compares a clump of stem cells to a ball sitting at the top of the hill.
“We give a set of instructive cues to the cells. We nudge the ball,” he said. “Once we set them down the path, they have an intrinsic ability to keep moving that program forward and give rise to these more complex structures.”
As with embryoid researchers, the organoid crowd doesn’t produce whole organs at this point (although they’d like to). One or two cues can set the stem cells on a particular path, but they veer off if they don’t continue to get the right signals. So how do you engineer a system that can continue sending the right signals?
It isn’t easy. Like organoids, each embryoid system has shortcomings because it’s not getting signals from tissues expected to be nearby. All of the researchers consulted for this story are interested in pursuing those next signals to keep the stem cells on track to model human embryonic development. And while they all have personal lines they wouldn’t cross, none of them believe they should be in charge of deciding where to stop.
“I don’t think people are really that concerned about making liver models or heart models in a dish,” said Hyun. “But artificial gonads, synthetic embryos, synthetic brain-like interfaces…we need to carve out some new rules for working with these novel entities.”
A popular argument for embryoids having no moral status – or limited moral status – is that without the extraembryonic support tissues, there is no potential for the stem cell model to develop into a baby.
“What’s a human embryo? Something with the potential to become a human,” said Siggia. “None of these things could become a human.”
Yet for someone inclined to see the epiblast – the future fetus – as the only part that matters, Hyun says, “Some people may view it as a baby that can’t attach to the womb – like a little astronaut in space without the spaceship. Some may say that’s still a person. It just doesn’t have the right support system.”
Both this argument and the no-potential argument ignore the critical role of the ultimate support tissue: the mother. Without her – the risks to her life and health, and the permanent damage to her body – there is no potential for even a complete and healthy embryo. Unless, of course, we do want to culture embryos all the way to full term. But no one is talking seriously about that.
What they want to do, as Hyun explained, is to model just enough to answer difficult questions.
The conversation started in academia, but carving out the space for embryoid research will mean getting input from a broader range of people. Sometimes, it is still the loudest voices that win the day. The reason embryo research in the U.S. can’t be funded with federal tax dollars is because of a vocal minority. Already, the National Institutes of Health recently declined to fund new embryoid studies.
It can also be hard to get people who should have a say to come to the table at all. In the case of infertility, the pain and stigma of repeated early miscarriages may make people less likely to advocate for research that could improve assisted reproduction.
“A couple who are having difficulty getting pregnant are usually very reticent about standing on the rooftops and shouting about their problem,” said Alison Murdoch, a professor of reproductive medicine at the University of Newcastle, UK, and advocate for embryo studies in fertility research.
Murdoch’s experience was very different when she was getting approval to prevent a serious genetic disorder with three-parent in vitro fertilized eggs. Parents and people living with congenital health problems are often willing to campaign for research.
“A parent whose child is dying is going to shout very loudly for help,” she observed.
The problems of two to six weeks after conception – the key period that future embryoids could explore – run between these extremes. But even if people are willing to talk, it’s not easy to get a representative committee together, said Hyun. Everyday people who are interested may not be able to take time away from their jobs to participate in a symposium or contribute to a report.
Now that Fu has designed a system that can generate thousands of early embryoids for purposes like drug screening, and other approaches are looking past the equivalent of 14 days, this is the time to have the conversations about what we want out of this research – and what we don’t.
Within the research community, Hyun has engaged Fu’s team and the Harvard Medical School team to begin developing a framework for bioengineering ethics in this new field. They are collaborating on a grant from the Greenwall Foundation, which funds bioethics research, to identify problematic structures and come up with ways to limit them.
That latter challenge, Hyun observed, can be trickier than anticipated. It was assumed that stem cells derived from an adult’s body could never go all the way back to pre-embryo states and form extraembryonic support tissues. But recent work suggests that they can.
“Biology can always surprise you and be more organized or more lifelike than you originally thought it was capable of becoming,” he said. In fact, that’s how Fu and Gumucio’s team ended up making an embryoid in the first place, when all they expected was amnion.